THE CENTER FOR RESEARCH INNOVATION
THE SPARK FUND
FUNDING NORTHEASTERN’S TRANSFORMATIONAL TECHNOLOGIES
The CRI Spark Fund helps Northeastern researchers bridge the gap between promising lab results and demonstrating a commercially viable prototype. The CRI grants and programs catalyze state-of-the-art technologies, advancing Northeastern inventions through prototyping, validation, and industry input.
OVERVIEW
$50,000 for one year towards professor led research
5+ awards available
The CRI Spark Fund offers proof-of-concept gap fund awards to commercially valuable inventions (from any field) in the early stages of development.
Spark Fund applicants may apply to advance a technology or suite of technologies towards commercialization readiness to:
- License either through new company creation or industry
- Apply for follow-on state, federal, or corporate funding to continue proof of concept work
New applicants applying for funding may only have one active Spark Fund award at a time. While you may apply to advance multiple technologies, you will only receive funding to advance one technology or suite of technologies at a time.
Prior recipients of Spark Fund or GapFund360 awards may apply for additional funding up to $150,000 maximum on a technology or suite of technologies. One must use active award funding for a full year prior to applying again.
Partnerships for Distinctive Awards:
These colleges collaborate with the CRI to provide financial support to emerging researchers. Researchers in other institutions receive funding exclusively from the CRI.
- Bouvé College of Health Sciences – Award dedicated to advancing research from the lab of a Bouvé Professor.
- College of Science – Award dedicated to advancing research from the lab of a COS Professor.
- College of Engineering – Award dedicated to advancing research from the lab of a COE Assistant/Research Professor.
DELIVERABLES
APPLICATIONS OPEN FALL 2024
SPARK FUND USES
Spark Fund grants may be used to advance the commercial prospects of inventions in many different ways, including but not limited to:

Building a commercially ready prototype

Animal testing to generate in vivo data

Optimizing processes for yield, speed, cost

Repurposing tech to meet industry needs
SPARK FUND BUDGET
- Salaries for research faculty
- Postdoctoral researchers, Ph.D.
- Graduate and undergraduate students
- Materials, supplies, travel expenses excluding conference attendance, and equipment and supplies necessary for the performance of the research provided that equipment costs do not exceed $2,000
- Costs related to animal care and research
- External consultant or advisor costs approved by the Spark Fund.
- Overhead or indirect costs
- Tuition
- Visa fees
- Scholarships
- Sabbatical leave
- Salaries for staff, visiting faculty, visiting researchers, tenured or tenure track faculty (including but not limited to the Lead Researcher) and other personnel not named in Allowable Expenditures above
- Conference attendance and travel
- Patent expenses
- Entertainment
- Equipment in excess of $2,000
- Sales, marketing, or other business development
- Debts, fines, or penalties
ELIGIBILITY
Applicant criteria
- Applicants must have an invention disclosure submitted to the CRI, and the CRI must have an initial patent application on file to be considered for Spark Fund.
- If you do not currently have an invention disclosure and CRI has not filed an initial patent application, please submit your invention disclosure and apply for the Spark Fund during the next funding cycle.
- Northeastern University researchers with novel, pioneering, and commercially valuable technologies in the proof-of-concept stage.
- Principal Investigators (PIs) on Spark Fund applications may include faculty, PhDs, Postdocs, or graduate students propelling inventions towards commercialization at Northeastern.
- At least one Co-PI must be a faculty member with Northeastern University IP who will oversee project progression and awarded funding.
IP criteria
- All technology affiliated with a Spark Fund application must be disclosed to the CRI and an initial patent application must be filed before applying.
- Spark Fund Eligible applicants include Northeastern University Inventors with novel, pioneering, and commercially valuable technologies currently in the proof-of-concept stage.
- All technology affiliated with a Spark Fund must be disclosed to the CRI before applying (including background intellectual property required to deploy the technology).
- At least one Northeastern Inventor with the duty to assign must either lead or oversee the proposed project.
- Awarded funds will be distributed to a Northeastern research lab.
Questions about eligibility? Contact Spark Fund.
ADVISORS
Advisors offer time and talent to review Spark Fund applications, sharing specific subject matter expertise and experience to enable effective selection of finalists and awardees.
FREQUENTLY ASKED QUESTIONS
CONTACT

PJ DUPUIS
ASSISTANT DIRECTOR OF MARKETING & PROGRAMS
Center for Research Innovation
(617) 373-8103
[email protected]

Safa Jamali, COE
Rheopoint
The Jamali lab is advancing Rheopoint, a science-based AI solution for engineering applications with focus on fluid mechanics and soft material design and discovery. Customer industries range from consumer products, to chemical and pharmaceuticals, and oil and gas.
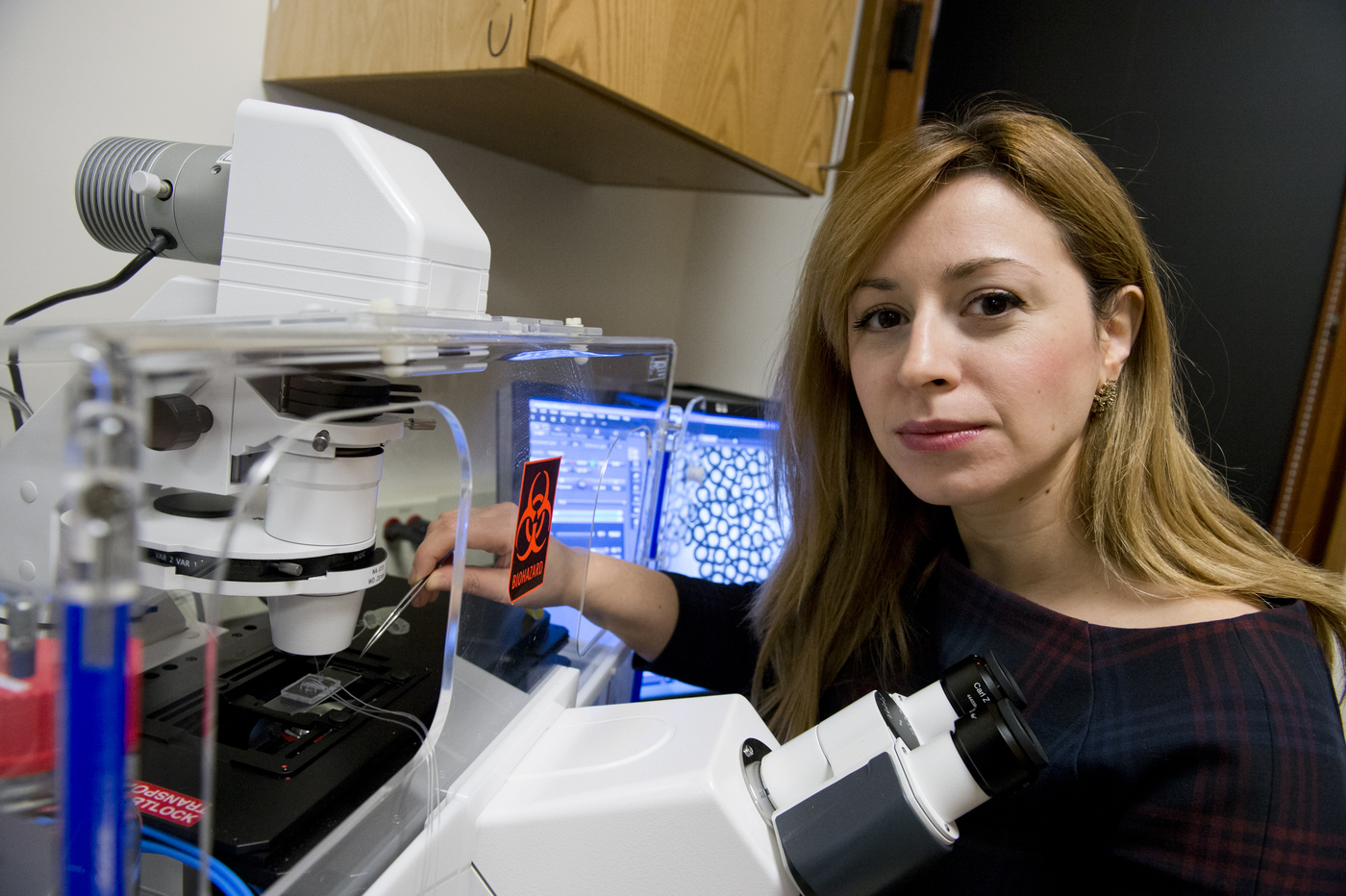
Tania Konry, BOUVE
Pathogen POCT
Konry Lab produces rapid, low-cost, highly accurate, and readily available point of care testing (POCT) solutions for microbial pathogen detection.

Tomasso Melodia, COE
CellOS
The Melodia lab is developing CellOS, a data-driven network operating system to automate the control of 5G/6G cellular networks and maximize their performance. CellOS combines softwarization and virtualization principles, network slicing and intent-based networking with data-driven solutions to automate network control and deliver high-performance services to mobile users.

Sara Rouhanifard, COE
ViralNPQ
The Rouhanifard and Wanunu labs are refining ViralNPQ, a cost effective and easy-to-use device that collects all viral measurements using the same sample. This solution images and quantifies the presence of a viral genome with single-molecule accuracy.

RAYMOND FU, COE
Spinout: Pathfind
Pathfind uses patented deep learning model compression techniques to transform once costly AI algorithms into customized lightweight processes. Pathfind enables complex computer vision and machine learning programs to run in real time on simple devices – bringing cutting-edge innovation to telemedicine, in-home fitness and other markets.

Neel Joshi, COS
Spinout: Tantu Therapeutics
Tantu is developing innovative microbe-based therapeutics expressed and delivered to the site of intestinal lesions to treat Inflammatory Bowel Disease (IBD) and other gastrointestinal diseases without systemic side effects.
